BACKGROUND
Clinical outcomes for B-cell Acute Lymphoblastic Leukemia (B-ALL) have significantly improved in the last decade, owing to the introduction of newer agents such as inotuzumab ozogamicin (INO) and blinatumomab. Both of these antibody-based therapies are effective in adults with relapsed or refractory (R/R) B-ALL and have shown promising results in the frontline setting as well. Given the utility of these therapies, there have been efforts to identify predictive markers for these treatments in B-ALL patients. Recently, CD58 was identified as a modulator of response to blinatumomab due to its role as a CD2 ligand in T-cell activation (Li et al. Sci Adv 2022). This costimulatory relationship between CD58 and CD2 has also been linked to Natural Killer cell mediated lysis, which has been described as a mechanism of action of INO (Fu et al. Signal Transduct Target Ther 2022; Huntington et al. Nat Rev Cancer 2020). In this study, we evaluated the correlation of CD58 expression with achievement of morphologic Complete Remission (CR) or Complete Remission with incomplete blood count recovery (CRi) after treatment with INO.
METHODS
Retrospective chart review was performed to identify patients in our institution's B-ALL database who received INO between 09/15/2011 and 03/10/2023 with blood or bone marrow samples available for flow cytometry analysis prior to INO infusion. Because all patients had measurable CD58 expression by flow cytometry, mean fluorescence intensity (MFI) was used instead of percent positivity to perform a simple logistic regression analysis to evaluate the relationship between CD58 MFI and achievement of CR/CRi after treatment with INO. We then calculated the odds of achieving CR/CRi by age, molecular risk at diagnosis, and R/R disease status. Two patients lacked diagnostic cytogenetic data. Molecular risk stratification and assessment of morphologic response were performed according to National Comprehensive Cancer Network (NCCN) guidelines. To account CD58 MFI outliers, we then confirmed our results by comparing response rates between patients with a CD58 MFI above our median (CD58 MFI-high) to patients with a CD58 MFI below our median (CD58 MFI-low) using Fisher's exact test.
RESULTS
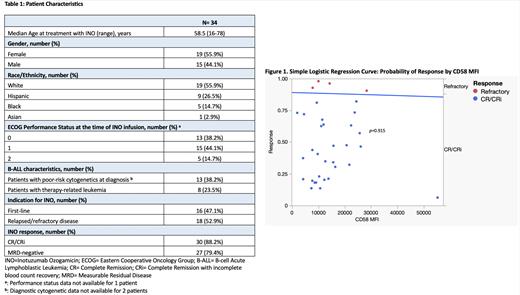
We found 34 patients who met our criteria for analysis, 2 of which had previously received INO (Table1). Median Overall Survival (OS) was 21.5 months (range, 1-58) with a median leukemia-free survival (LFS) of 13.5 months (range, 1-52). Response rates after INO were high, including 28 patients (82.4%) achieving CR, 30 patients (88.2%) achieving CR/CRi, and 27 patients (79.4%) achieving Measurable Residual Disease (MRD) negativity. Both patients with a history of prior treatment with INO attained CR with MRD negativity. The chi-square test statistic for our simple logistic regression model was not statistically significant (Figure1, p=0.915). The logistic regression coefficient showed no statistically significant association between CD58 MFI and CR/CRi rates after treatment with INO ( p=0.913). Our model predicted an 88.6% probability of achieving CR/CRi for a CD58 MFI of 8,727.97 (Q1), 88.4% for 11,719.88 (Q2), 88.0% for 19,622.85 (Q3), and 85.7% for 55,217.74 (Q4). There was no statistically significant difference in the odds of achieving CR/CRi in patients with poor molecular risk at diagnosis (OR=0.06, 95% CI [0.01, 1.22]), age >35 years (OR=2.75, 95% CI [0.33, 22.9]), or R/R disease (OR=0.98, 95% CI [0.01, 1.97]). When comparing response rates between CD58 MFI-high and CD58 MFI-low patients, each group contained 15 patients that achieved CR/CRi and 2 patients with refractory disease. This confirmed an equal response rate to INO between CD58 MFI-high and CD58 MFI-low patients ( p=1.000).
CONCLUSION
Overall, we did not find a statistically significant correlation between CD58 MFI and achievement of CR/CRi. Our study is limited by our small sample size, single-center location, and retrospective design. However, it is important to note the high rates of CR/CRi that were seen, regardless of the intensity of CD58 expression. Further work is needed to evaluate the role of CD58 as a predictive marker for other therapies in B-ALL. These data suggest that INO is likely to remain an effective option for these patients, regardless of the intensity of CD58 expression.
OffLabel Disclosure:
Stock:Servier: Other: Data Safety Monitoring Board/Advisory Board; Newave: Honoraria; Kite: Consultancy; Kura: Research Funding; Jazz Pharmaceuticals: Consultancy, Honoraria; Glaxo Smith Kline: Consultancy; Amgen: Honoraria. Gurbuxani:UpToDate: Patents & Royalties: Royalties for contributions to various topics; AbbVie: Consultancy; Jazz Pharmaceuticals: Consultancy. Patel:AbbVie: Honoraria; Kronos Bio: Research Funding; Pfizer: Research Funding; BMS: Honoraria.
Inotuzumab ozogamicin is currently approved for relapsed/refractory B-ALL. We will include response rates of patients treated with inotuzumab as a first-line of therapy.